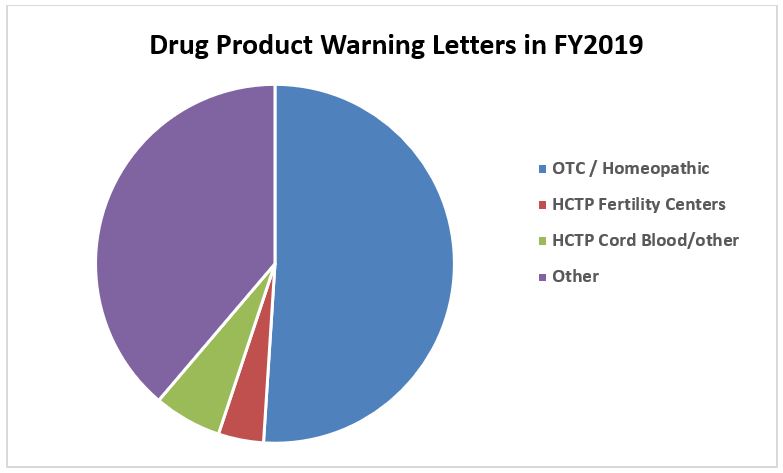
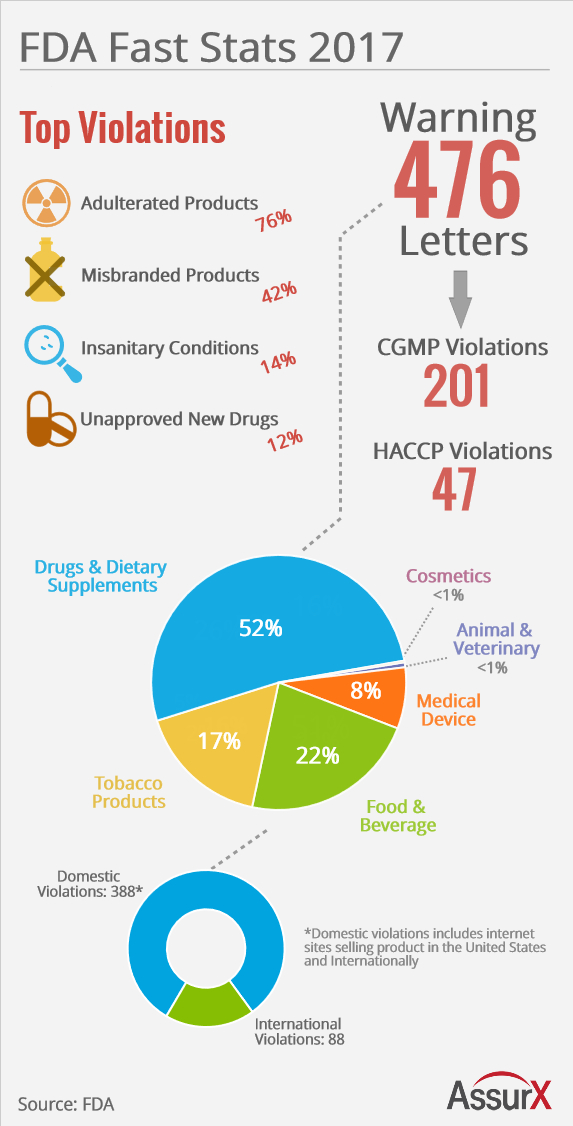
FDA Urges Contamination Control in Warning Letters for Dominican, Indian Drug Manufacturing Faciliti | RAPS
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF NEW JERSEY CAMDEN VICINAGE In re: Valsartan NDMA Products Liability Liti
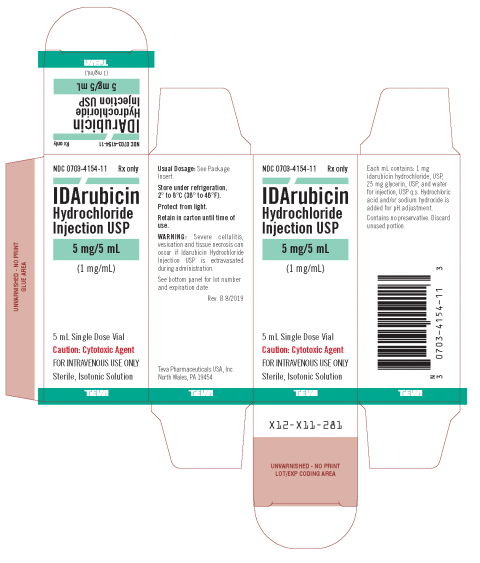
Teva Issues Voluntary Nationwide Recall of One Lot of IDArubicin Hydrochloride Injection USP 5 mg/5 mL Due to the Presence of Particulate Matter | FDA

Teva faces drug price fixing charges, to cut 350 jobs in Israel; Wintac gets hit by FDA Warning Letter | Radio Compass Blog

AdComplyRx POV: March 3rd, 2020: FDA Warning Letter Specific to Paid Search (SEM) — MyAdComplyRx - Pharma SEM Regulatory & Brand Safety